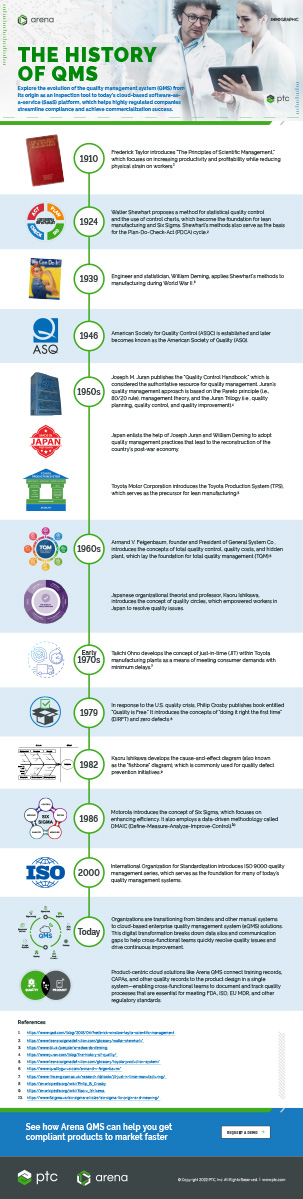
HISTORY OF QMS
Quality management was first introduced in medieval Europe when craftsmen guilds developed strict guidelines for inspecting products.
In the late 19th century, mechanical engineer Frederick Winslow Taylor broke away from the traditional European craftsmanship model and quality practices to develop a new approach which focused on increasing productivity without increasing strain on workers. This led to his publication of “The Principles of Scientific Management” in 1910.1
In the 1920s, engineer Walter Shewhart developed statistical quality control methods to help businesses reduce variation and streamline production. His methods (also known as the Shewhart Cycle) were applied to the production of military goods during World War II, enabling armed forces to enhance product safety and quality. The Shewhart Cycle also served as the basis for the Plan-Do-Check-Act (PDCA) cycle, which is a key component of many of today’s quality management systems.2
Throughout the 1950s and 1960s, Japan focused on quality in an effort to rebuild its economy after the devastation of World War II. Japanese manufacturers soon adopted a total quality strategy which held all workers accountable for improving operational processes. This total quality approach enabled Japan to produce increasingly higher quality products at lower prices and resulted in an economic boom in the decades that followed.3,4
Around the same period, Japanese car manufacturer Toyota introduced the Toyota Production System (TPS). It was the precursor to Lean manufacturing which focuses on increasing productivity while minimizing waste.5
In the 1980s, American business leaders started to embrace the total quality management (TQM) movement in an effort to compete with Japan. TQM provided them a framework for implementing effective quality and production processes across their organization.
As the fundamentals of TQM started to fade, new quality management initiatives started to emerge. And in 1986 Motorola introduced a quality control method called Six Sigma.6 Since then, many organizations have adopted Six Sigma to increase profitability.
A year later, the International Organization for Standardization published the ISO 9000 series of quality management standards. These standards were designed to help companies document and manage the various elements of a quality management system so that they could increase customer satisfaction, meet regulatory requirements, and drive continuous improvement.
Today, companies are moving away from folders, binders, and other manual systems and embracing enterprise quality management system (eQMS) software to better manage and track all the processes and records that are tied to their quality system.