When Do You Need a Quality Management System?
All our customers, no matter industry, maturity, or complexity, have had to answer the same question: when to implement a scalable software solution to manage the enterprise processes that run the businesses.
Every company has more on the “to do” list than resources. You have many business needs—ranging in scope and impact—and a finite pool of people and budget that will not cover all the business needs. What does this mean? You’ll need to prioritize your projects. And, if you are a medical device company, one of these projects will be a quality management system (QMS) solution to meet the compliance requirements of FDA, EU MDR, and ISO at scale. Most life sciences companies start with paper because you must document what you are doing.
Additionally, medical device companies need to grapple with when to implement a product lifecycle management (PLM) system because one of your business requirements is to bring new features and products to market in a predictable, efficient way. PLM helps you do this.
How do you prioritize this project against all the other projects, particularly when you are a younger company with too much to do? How long can you get by with Excel, Dropbox, email, and Zoom calls? Let me share some research from our customers that will help.
Not Covered Here: Software Requirements, Business Value, TCO, ROI
Before we start, a disclaimer. In this blog, we aren’t going to talk about the specific business needs or processes that QMS supports, the requirements you need to define for a solution, the business value a good solution should provide, or total cost of ownership (TCO) evaluations and return on investment (ROI) calculations. These are all important considerations you need to address ONCE you decide it is time for any system, including QMS. We are going to talk about what should determine the best time for you to put a scalable, purpose-built platform in place—instead of the spreadsheets or square-peg tool you might be using today.
Timing Drivers
We work with a lot of product companies—startup to large; across many industries; and with a range of products in volume, price, and complexity. We track what drives our customers to buy a solution at the point in time they do—their timing drivers.
A timing driver is the reason you will prioritize this project above other projects right now and is the combination of company stage, upcoming product events, company culture, and recent experiences.
What are timing drivers? For our customers, we’ve identified five primary timing drivers:
- Proactive pain avoidance
- Compliance
- Growth
- Gross inefficiencies
- Upcoming new product development and introduction (NPDI) events
While these drivers as categories have held steady over the years for our customers, how much each driver shows up has varied across years, given the overall economic, competitive, and political climates. Here we’ll look at our 2020 Arena freshman class of QMS customers to see the importance of each driver in this season.
- Proactive Pain Avoidance
Interestingly, this driver is the most-often cited in 2020 for all our customers, not just those in life sciences or those shopping for a quality management system. Proactive companies are typically companies with experienced team members who have “been there, done that” and know that they need to put certain systems and processes in place before costly mistakes occur due to errors or challenges when they begin to scale. For life sciences companies, even in 2020, you need to prepare for what is coming—compliance, commercialization, and scale.- Compliance
For medical device companies, compliance is always top-of-mind. Compliance is a timing driver, focused usually on upcoming events, such as 510(k) submission, premarket approval (PMA), EU Medical Device Regulation (MDR) assessment, external audits, certifications, and other regulatory filings. You might expect compliance to be a more frequent timing driver than the 35% reported, but proactive pain avoidance often includes being proactive for compliance, according to the customers we interviewed. In companies with multiple timing drivers, compliance is the top driver, reinforcing that as companies grow and plan for continuous quality, they recognize that the systems must ensure compliance at the same time.- Growth
Times of growth create good problems for companies to solve as existing tools, processes, and methods need to be reviewed for update and replacement. While 2020 has seen falling revenues for some companies, in many life science markets the demand has exploded while for others it has remained steady. Think of COVID-19-related analysis and treatment, health and wellness, sanitation and disinfection, and almost anything that makes your abode a home.- Gross Inefficiencies
If you are doing QMS activities with a mix of generic tools, you’ll have inefficiencies at best, errors at worst. Inefficiencies usually function as a cost of your current way of work, but the work still gets done even if you use a specialized (high-cost) team that could be doing more valuable work. However, for some companies, inefficiencies are too painful due to extremely limited resources (people). The result is that you are not able to get work done anywhere close to on time. In this situation, we have gross inefficiencies becoming a driver of when to make the switch, not if.- Upcoming NPDI Events
For all product companies, new product development and introduction (NPDI) happens. While NPDI is a routine part of progress (growth), you might have a particularly high-profile product commercialization in the future or multiple product projects with complexities (expanded supply chain, new markets, new revenue goals, competitive play) that require you to put a QMS solution in place to reduce risk and be ready to handle customer complaint and corrective and preventive action (CAPA) processes.Importance of Quality Management System Timing Drivers
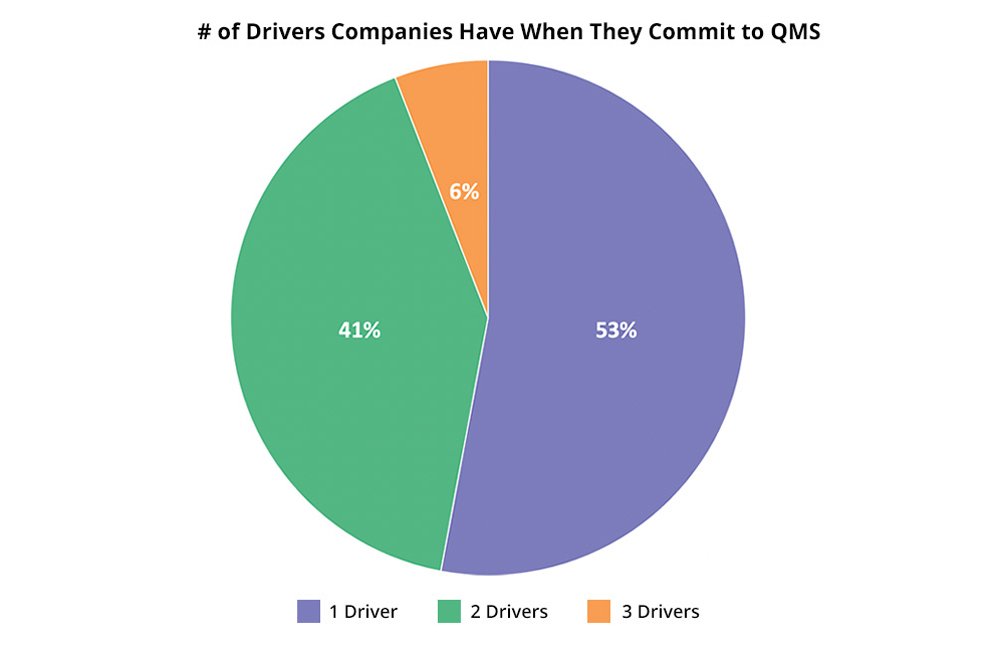
In working with our customers for decades, we’ve learned that the more timing drivers they have, the more likely they will be able to get the budget, resources, and political will to implement a QMS solution. While 53% of our 2020 new QMS customers had only one primary timing driver, 47% had two or more.
More importantly in QMS customers with more than one primary timing driver, proactive pain avoidance and compliance are the most frequent and are forward-looking, positive drivers that indicate many life sciences (and other strongly regulated industry companies) make systems a priority earlier rather than later.
Determine Your Quality Management System Timing Drivers
You will want to have conversations with your team about what is going on in your company: your quality processes, product development and manufacturing projects, company goals, other projects competing for resources, and the company culture. Gaining a big picture of what is going on over the next time period will help with your QMS solution timing.
And, if you are a medical device company, you should consider QMS drivers in conjunction with PLM drivers as the processes are intertwined and silos of data will increase inefficiencies and potentials for errors and regulatory failures. We’ve got the QMS + PLM solution you need.
Remember: A timing driver is the reason you will prioritize this project above other projects right now and is the combination of company stage, upcoming product events, company culture, and recent experiences.
Once you have the big picture, you’ll identify your QMS timing drivers.
Here is a quick scoring card along with common (and real) examples from Arena customers for each driver.
✔
Timing Driver
Examples
Proactive Pain Avoidance
- We want to do this right from the beginning
- We are changing our supply chain model significantly—more/less in-house, new supply chain partners, more supply chain partners, etc.
- We don’t want to invest in building our own solution, expanding our IT department, rolling out the paper process for new people
- We know we will be going for a big goal in future (510k, PMA, ISO 9001/13485/14971, 8D, 510K, merger) and we want something in place sooner rather than later
Compliance
- We will commercialize products in multiple geographic markets that require additional regulatory management
- We have a filing or audit in six months and must ensure compliance
- We failed an audit, standard, or regulation
Growth
- We’ve doubled our team in the past six months
- We have five new products coming out next year
- We’ve won a significant contract and now need to execute
- We’ve acquired a company
Gross Inefficiencies
- Everyone went #WFH in March and everything now takes too long or is too hard
- Quality resolutions are time-consuming
- We need to streamline and speed up product development
- People are doing the same things repeatedly and disconnected from each other
Upcoming NPDI
- We plan to commercialize our first product in nine months
- We have a second product effort starting
- We are launching a new product line
- We are expanding into a new market
Take Action Now or Later?
You should be ready to have conversations as to if QMS is a priority project or if your company can wait. The right time to implement QMS is unique to your company culture, ways of work, product specifics, and business goals. Sometimes, even though you need a solution, the time isn’t right. Twenty percent of our new customers choose Arena after multiple discussions with us.
If you marked only one timing driver, you’ll want to look at the other projects vying for resources and their timing drivers. You may need to construct a business-needs statement and capture the cost of how you do work today. Inefficiencies, inaccuracies, missed communications, and lack of collaboration can add up to excessive costs. If you are currently running quality management on spreadsheets, jump over here to explore the risks. If your one timing driver is compliance, this effort probably should win over all others as meeting regulatory requirements is the minimal must-have before you can improve and scale.
If you have two or more drivers for QMS now, this is the time to move QMS to the top of the list. Congratulations—with two or more drivers, you must have exciting work planned that can leapfrog you into the market or over competition. Below are some great resources to start team conversations about your possible future.
- Proactive pain avoidance—7 Principles of Product-Centric Quality Management
- Compliance—Strong Design Controls Simplify Compliance
- Growth—Innovation for a New Era
- Gross inefficiencies—Getting to Milestones Practical Guide
- Upcoming NPDI—Connecting Teams and Data in NPDI
- Streamlining Environmental Compliance to Foster Sustainable Product Development