“Design Input Requirements” are critical components within the domains of Quality Management Systems (QMS) and Product Lifecycle Management (PLM), serving as a point of reference for product development starting from blueprint to market. These QMS requirements guarantee that products adhere to regulations, meet quality standards, and satisfy customer expectations. Performance criteria, safety standards, and functional specifications are integral components that ensure the consistent quality of the product during the entire development process.
Design input requirements comprise PLM’s comprehensive specifications for the functionality and performance of a product; they inform design and development decisions. Ensuring quality and regulatory compliance while addressing market demands, these requirements are critical for innovation.
It is critical to incorporate design input requirements into QMS and PLM. They facilitate systematic quality management in QMS and direct the product lifecycle from conception to disposal in PLM. By integrating quality and regulatory standards with innovation and market alignment, this process strikes a balance between efficiency, effectiveness, innovation, and compliance during product development.
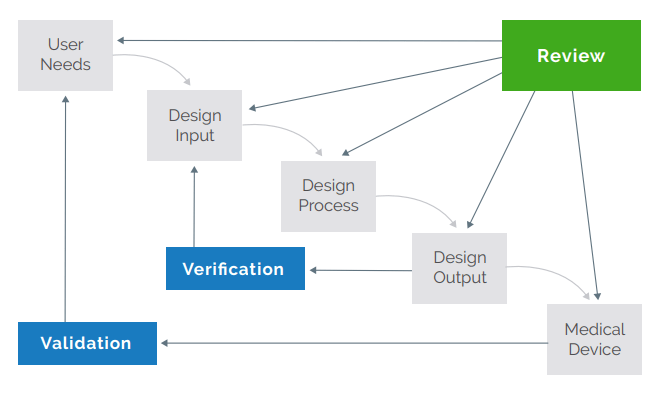
Read how Arena’s Traceability Matrix and other expanded QMS capabilities help manufacturers overcome common product development challenges.
