The new IVDR has expanded labeling requirements to increase transparency and traceability of medical devices. These changes will most likely impact manufacturers’ labeling processes and may require the use of different labeling equipment.
Device labeling must include:
Device name and trade name
Standardized symbol to indicate that the product is an in vitro diagnostic medical device
Package contents and intended purpose
Contact information of authorized EU representative (for non-EU based manufacturers)
Unique device identifier (UDI)
Time limit for using the device safely (i.e., year and month)
Warnings and precautions related to the device
Serial number and lot number
Links to electronic instructions for use (eIFUs) and company website
Indication of any special storage and/or handling conditions
Indication of sterile state of the device or any special microbial state
Indication if the device is intended for single use
Indication if device is intended for self-testing, or near-patient testing
Type of specimen(s) required to perform the test (e.g., blood, urine, saliva)
Arena QMS Tip:
Change Order Custom Attributes
When initiating change orders for product labels, create a change order custom attribute for impact analysis to help identify all instances where the addition of a unique device identifier (UDI) and other labeling changes will apply.
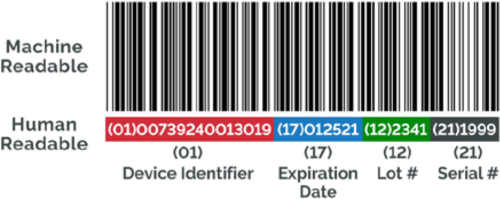 The unique device identifier (UDI) requirement is a significant change that has been introduced under the new regulation. Comprised of a series of numeric or alphanumeric characters, the UDI allows for the unambiguous identification of specific devices on the EU market and enables better traceability and monitoring by authorities. In addition to being added to device labeling, the UDI information must also be listed on the declaration of conformity and uploaded to the European database for medical devices (EUDAMED).
The unique device identifier (UDI) requirement is a significant change that has been introduced under the new regulation. Comprised of a series of numeric or alphanumeric characters, the UDI allows for the unambiguous identification of specific devices on the EU market and enables better traceability and monitoring by authorities. In addition to being added to device labeling, the UDI information must also be listed on the declaration of conformity and uploaded to the European database for medical devices (EUDAMED).
All in vitro diagnostic medical device labels should be presented in a human-readable format. As such, manufacturers may need to increase the sizes of labels or use internationally recognized symbols (per ISO 15223-1) to accommodate more information.
Section 2: Key Changes Under New IVDR
Economic Operator Roles and Responsibilities
Performance Evaluations, Performance Studies, and Post-Market Performance Follow-up
Post-Market Surveillance (PMS)
European Database for Medical Devices (EUDAMED)
Section 3: Quality Management System (QMS) Requirements
Section 4: Steps For A Successful EU IVDR Implementation