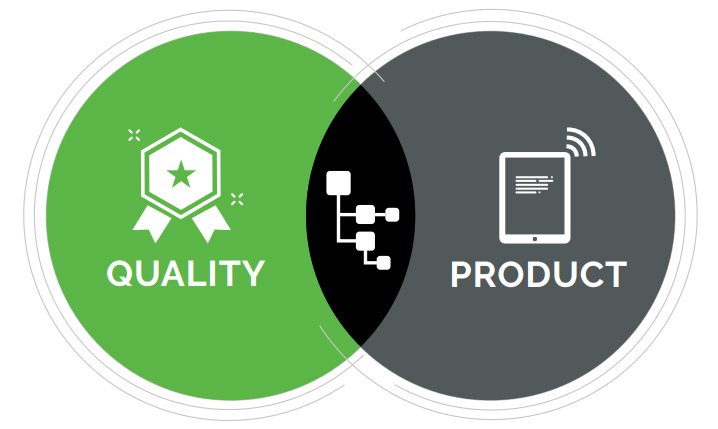
For medical device companies, document control is critical to success. Despite that absolute, traditional stand-alone QMS systems address only a subset of what’s needed, focusing only on two fundamental areas of FDA compliance:
- Documentation control for SOPs, specifications, and other files
- Process enablement and tracking for auditable processes (including CAPA, requirements management, and training records)
While the automation of paper-based processes is helpful, that function alone fails to capture or connect quality management processes to the comprehensive product record often comprised of hundreds if not thousands of mechanical, electrical, and software components; assembly and test procedures; and other documents specified in a product’s bill of materials (BOM).
Traditional document-centric QMS approaches may suffice for some life sciences companies that don’t have complex products. However, medical device companies that develop products with a mix of electrical, software, and mechanical components must bring crossfunctional teams and designs together to prevent product issues from arising too late in the NPI process. They must also provide traceability between all aspects of the design and quality elements and be able to track, control, and release product design changes to market quickly and effectively. And, they must be able to address product failures to identify, analyze, and resolve issues. Let’s look deeper. Read more about Determining the right type of quality management system for your needs.