How Medical Device Manufacturers Can Avoid Recalls
Introduction

2018 was a difficult year for many medical device manufacturers, with large spikes in Class I recalls and a whopping 186,580,917 devices across 343 recalls affected in Q1. While the impact in Q2 and Q3 was lower, the number of recalls was still high. In fact, Q2 had the most single-quarter recalls since 2005, clocking in at 360 recalls affecting 42.4 million devices.
So, what gives? Why are Class I medical device manufacturers seeing this sharp increase in recalls? And more importantly, how can conscientious companies prevent them from happening in the first place?
Having the necessary quality controls is extremely important to prevent or respond to product recalls. Ensuring that there are proper quality management system (QMS) processes in place can help prevent FDA warnings and recalls. And, should a recall occur, the ability to refer to complete and transparent quality processes will help medical device manufacturers respond to recalls, provide necessary corrective actions quickly, and avoid harm to patients before the device manufacturer’s reputation is damaged significantly.
Issues with medical device software
Software issues are becoming more and more prevalent as medical devices grow increasingly connected and complex. Due to an increased interoperability reliance with electronics, software issues were to blame for nearly a quarter of medical device recalls in 2018. With the shift towards more software-intensive devices, manufacturers must prioritize the design and testing stages to catch product and software upgrade issues before devices ship to market.
Software problems can also pose potentially life-threatening risks if they’re not caught or addressed. For example, a company’s 3D brain imaging product for surgeons received a Class I recall due to a software defect that prompted incorrect values when switching between configurations within a particular third-party port. The malfunctioning software could cause serious injury or even death by hindering a surgeon’s ability to see accurate displays during surgery.
In order to identify any software issues before a device reaches a customer, manufacturers must conduct extensive and accurate testing throughout the new product development and introduction (NPDI) process. Using a product-centric QMS solution that enables collaboration processes between engineering, quality, and manufacturing teams help accelerate product launch while ensuring devices work as designed.
Documentation gaps
Incorrect or incomplete documentation is another large contributor to medical device recalls. As devices are tested and issues identified, design documents are modified, and all associated specifications, procedures, training plans, and other related materials are updated. Given the evolving nature of the design and revision process, documenting and ensuring clear communication of all changes made once devices are in the hands of doctors, patients, or hospitals can be difficult.
A company that produces devices that heat and cool patients during surgery experienced this burden firsthand when it needed to revise its cleaning instructions and communicate those changes to customers. These revisions were made in response to the FDA’s three-year investigation from 2015-2018 of a bacterial risk associated with heater-cooler devices, after the devices had been manufactured and delivered for 20 years.
The ability to document and test how devices are intended to work can help prevent these types of incidents before products are shipped. Many small medical device manufacturers create patch-quilt systems involving generic cloud apps and file-sharing solutions. These are not intended to address product design or quality issues, nor ensure the proper controls as you move through the NPDI process. Larger, more mature medical device companies that have grown rapidly are even more susceptible to relying on disparate legacy tools for documenting product design and quality issues. These siloed tools make it difficult, if not impossible, to see the relationship between product designs (including parts, bills of materials or BOMs, software, electrical, and hardware components) and quality processes.
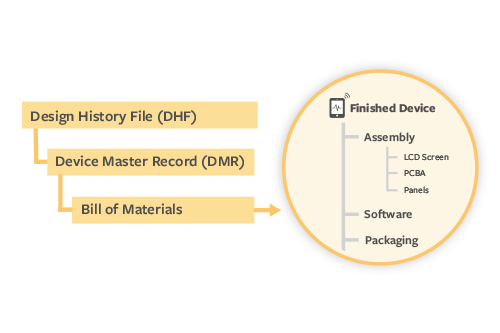 With Arena’s product-centric QMS, device manufacturers don’t have to rely on disconnected document-centric QMS and product development systems. Instead, Arena QMS can provide a single, scalable solution to manage the entire product record including items and BOMs, along with quality records like DMRs, DHFs, procedures, and all associated documentation.
With Arena’s product-centric QMS, device manufacturers don’t have to rely on disconnected document-centric QMS and product development systems. Instead, Arena QMS can provide a single, scalable solution to manage the entire product record including items and BOMs, along with quality records like DMRs, DHFs, procedures, and all associated documentation.
Problems with medical device manufacturing
Manufacturing issues can happen for multiple reasons, including design errors, non-compliant materials, unclear manufacturing instructions, and interoperability problems, to name just a few. Anticipating and dealing with manufacturing problems is daunting, and many manufacturers lack the necessary design controls or traceable processes to do so.
A company that makes implantable cardiac defibrillators demonstrated the severe impact of this issue when they were hit with a Class I recall in 2018. A manufacturing defect caused an out-of-specification gas mixture to form in the device, ultimately thwarting the electrical shock needed to restart a patient’s heart. This malfunction could have caused serious injury or death in some patients.
With complex products like these, having full visibility into the product record is imperative, so that everyone involved with designing, testing, and producing the device can collaborate effectively and increase the ability to identify issues before products are shipped to customers. Arena QMS maintains quality process links to every aspect of the product design, so product teams can instantly review product design records from components and multilevel BOMs to quality process records or vice versa. In this way, auditors, quality and regulatory affairs, engineering, manufacturing, and other affected groups can instantly review and evaluate designs and issues through final resolution in a single system of record.
Medical Device Recalls can happen to anyone – Don’t let it be you!
Given the common breakdown across these quality subsystems, increases in product recalls appear to correlate to the expanding complexity of medical devices and the interoperability of software. With the move towards advanced devices, it’s more important than ever to have an equally complex and comprehensive QMS solution that connects the quality and product record to improve visibility, controls, and traceable processes for everyone across internal teams and external supply chain partners.
Looking to safeguard your innovative device from product recalls down the road? Talk to one of our team members today about the product-centric advantage and improved compliance Arena QMS can provide.
Links:
Top 5 Causes of Medical Device Recalls
Why Have Medical Device & Drug Recalls Increased Dramatically in 2018?


