-
- North America
- EMEA
MMI (Medical Microinstruments, Inc.) was founded in 2015 in Pisa, Italy. MMI’s proprietary Symani® Surgical System combines the world’s smallest wristed microinstruments with tremor-reducing and motion-scaling technologies to address significant unmet patient needs across the globe. This first-of-its-kind surgical robotic platform for open, soft tissue micro-level surgery can help address microvascular repair, lymphatic repair, and peripheral nerve repair.
Utilizing a powerful combination of miniaturized instruments and robotic technology designed to perform microsurgery and supermicrosurgery, MMI’s Symani® Surgical System is one of the most significant transformational advancements in the field. The platform enables accurate micro-movements with motion scaling and tremor reduction designed to improve a surgeon’s ability to repair anatomical structures such as arteries and vessels as small as 0.3 mm in diameter. NanoWrist® technology, which is the smallest wristed instrumentation in the world, aims to enhance a surgeon’s natural dexterity and range of motion beyond what the human hand is capable of.
Arena PLM and QMS
Nimble, user-friendly, and easy to implement
The capacity to control and manage a vast amount of documentation and engineering changes while meeting the challenges of global medical device regulations
MMI is on a mission to advance robotic technology that pushes the limits of soft tissue open surgery and creates new opportunities for surgeons to restore the quality of life for patients with complex conditions.
As a small medical device company working rapidly to launch an innovative product, they needed to maximize efficiencies wherever possible. MMI identified an opportunity to streamline siloed design and development information to accelerate product compliance and commercialization as well as establish a solid foundation for growth.
Early on, the company had a fully functional platform. To achieve commercialization, MMI needed to meet rigorous compliance requirements. They knew a centralized system to manage the numerous components of a complex robotic system – mechanical, electrical, software and controlled processes – was critical to proving compliance and bringing a safe and effective product to market.
Product information was dispersed over multiple platforms, including eCAD, mCAD, PDM, quality management, and software management systems. These systems shared information manually which was not only time-consuming but prone to error. Likewise, communicating current and accurate information with contract manufacturers (CMs) was difficult. MMI knew it was time to implement a product lifecycle management (PLM) solution.

—Mauro Ercolani, Vice President of Quality and Regulatory Affairs, MMI
Joining MMI in 2018, Mauro Ercolani, Vice President of Quality and Regulatory Affairs, saw the company rapidly evolving. He immediately recognized the need for a PLM system to successfully meet compliance requirements and expand product capabilities. This would give the company a centralized location to manage engineering changes, bills of materials (BOMs), product documentation, and the ability to streamline collaboration between internal teams and external partners.
Upon joining the company, Mauro suggested Arena. His recommendation was based on Arena’s understanding of modern technology companies, especially when it comes to complex products and the compliance requirements of medical devices.
There was unanimous support for the recommendation, especially from MMI’s CEO who has significant experience with the software and a strong desire to implement it. Not only was Arena a proven cloud solution for medical device companies, but the system also provided a unified platform for PLM and quality management. Mauro said, “To me, Arena was the right move, a fundamental choice because any company that manages sophisticated products cannot live without PLM.”
Designed to be configured and deployed fast, Arena’s cloud-native PLM and QMS solution provides MMI and their supply chain partners with a business-ready application that can be accessed anytime and anywhere. “I was pleased to learn Arena’s philosophy to configure versus customize the system,” said Zeno De Luca, Arena Administrator for MMI. “In my experience, customizing a system can be difficult and time-consuming. The configurability of Arena allows me to focus on critical work and ensures seamless product updates.”
With Arena, MMI has been able to consolidate the product configuration across various systems, eliminating the need for paper-based processes. Arena’s cloud-native platform enabled the company to scale its Arena system from item and BOM management to change management, document management, and training management. “At the moment, these processes are successfully managed through Arena. We are excited about the opportunity to continually discover additional system capabilities that can benefit us,” explained Mauro.
With roughly 50 training plans in place using Arena Training Management, MMI can efficiently meet training requirements for compliance. The company can ensure training on all their technical documentation such as device master records (DMRs) and operating instructions to help eliminate nonconformities, CAPAs, and complaints, so any actions that need resolution can be assigned to the right teams or groups.
Selling their products globally means MMI must comply with European (EU MDR) and U.S. FDA medical device regulations, as well as any country where the company wants to do business. When it comes to providing necessary process controls for compliance and connecting quality processes to the product BOM for maximum visibility and traceability, Mauro said, “Going from research to validation to production, we have tailored the workflows of the change orders so that they match the regulatory requirement, and we keep the burden for the user at the minimum possible level while keeping full compliance with the regulations.”
Arena Validate has helped MMI meet FDA 21 CFR Part 11 and Part 820 directives for software validation by validating Arena’s applications against predefined guidelines such as change and document management, design controls, DHFs/DMRs, electronic signatures, and quality management. Zeno said, “With Arena, we have a validated system that allows us to comply with regulatory body directives. We don’t need to spend time and money validating the system, it’s already done.”
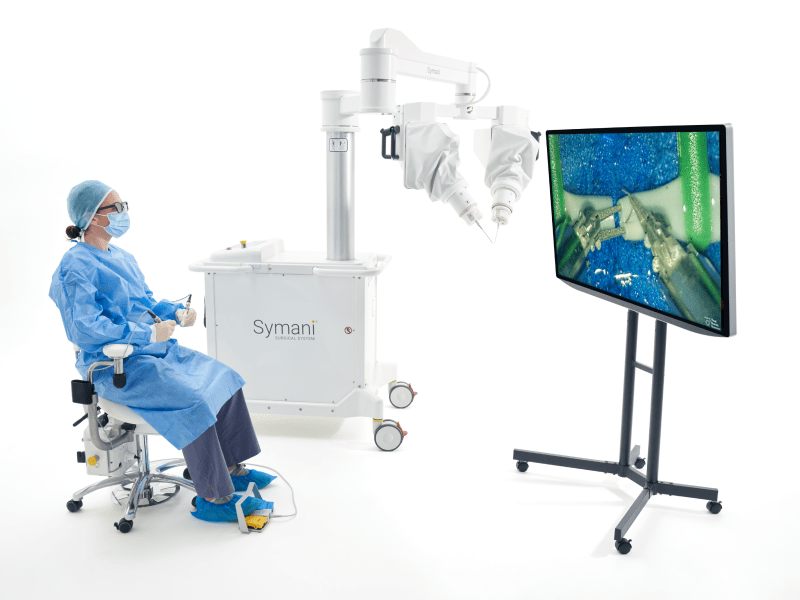
With the implementation of a cloud-native unified PLM and QMS solution, MMI has been able to move away from siloed systems and information and consolidate its product documentation, processes, and information into one single source of truth.
Audits and inspections are easier to manage because documents are easy to find and can be reviewed in real time to quickly prove their processes. “There’s no time wasted searching for a document and printing it to show the auditor,” noted Mauro.
MMI has been able to scale and take advantage of all that the connected PLM and QMS solution has to offer with Arena’s online resources and knowledgeable customer support team. Pleased with their local implementation and support from Arena, Zeno stated, “I think the development team is doing a great job in terms of facilitating the processes, execution, and the new upgrades. The team has been effective in responding to our needs.”
In just over four years since implementing Arena, MMI has executed countless engineering change orders (ECOs) and quality processes. “As our products have advanced over the years, Arena has enabled us to effectively manage hundreds of supply chain partners in addition to thousands of items and changes that otherwise would have resulted in massive inefficiencies and a negative business impact,” stated Mauro.
Looking ahead, MMI plans to integrate Arena with its enterprise resource planning (ERP) and product data management (PDM) solutions to ensure that the information is seamlessly shared across all systems to further automate communication between design and manufacturing, eliminate duplicate data entry, and reduce late-stage errors.
As MMI continues its mission to advance robotic technology and push the limits of soft tissue open surgery, the company can be confident that the solid foundation they’ve established with Arena PLM and QMS will help them answer the call to restore the quality of life for patients with complex conditions.
With the implementation of a cloud-native unified PLM and QMS solution, MMI has been able to move away from siloed systems and information and consolidate its product documentation, processes, and information into one single source of truth.
Audits and inspections are easier to manage because documents are easy to find and can be reviewed in real time to quickly prove their processes. “There’s no time wasted searching for a document and printing it to show the auditor,” noted Mauro.
MMI has been able to scale and take advantage of all that the connected PLM and QMS solution has to offer with Arena’s online resources and knowledgeable customer support team. Pleased with their local implementation and support from Arena, Zeno stated, “I think the development team is doing a great job in terms of facilitating the processes, execution, and the new upgrades. The team has been effective in responding to our needs.”
In just over four years since implementing Arena, MMI has executed countless engineering change orders (ECOs) and quality processes. “As our products have advanced over the years, Arena has enabled us to effectively manage hundreds of supply chain partners in addition to thousands of items and changes that otherwise would have resulted in massive inefficiencies and a negative business impact,” stated Mauro.
Looking ahead, MMI plans to integrate Arena with its enterprise resource planning (ERP) and product data management (PDM) solutions to ensure that the information is seamlessly shared across all systems to further automate communication between design and manufacturing, eliminate duplicate data entry, and reduce late-stage errors.
As MMI continues its mission to advance robotic technology and push the limits of soft tissue open surgery, the company can be confident that the solid foundation they’ve established with Arena PLM and QMS will help them answer the call to restore the quality of life for patients with complex conditions.

—Zeno De Luca, Arena Administrator for MMI