Traceability, velocity, and compliance are critical as you prepare your products for commercialization. Arena’s product-centric quality management system (QMS) solution is designed for highly regulated companies developing sophisticated products that rely on complex supply chains throughout their product lifecycles. Arena QMS connects quality records and product development information in a single, secure, cloud-native system, helping Medical Device companies introduce new products to market faster while ensuring regulatory compliance.
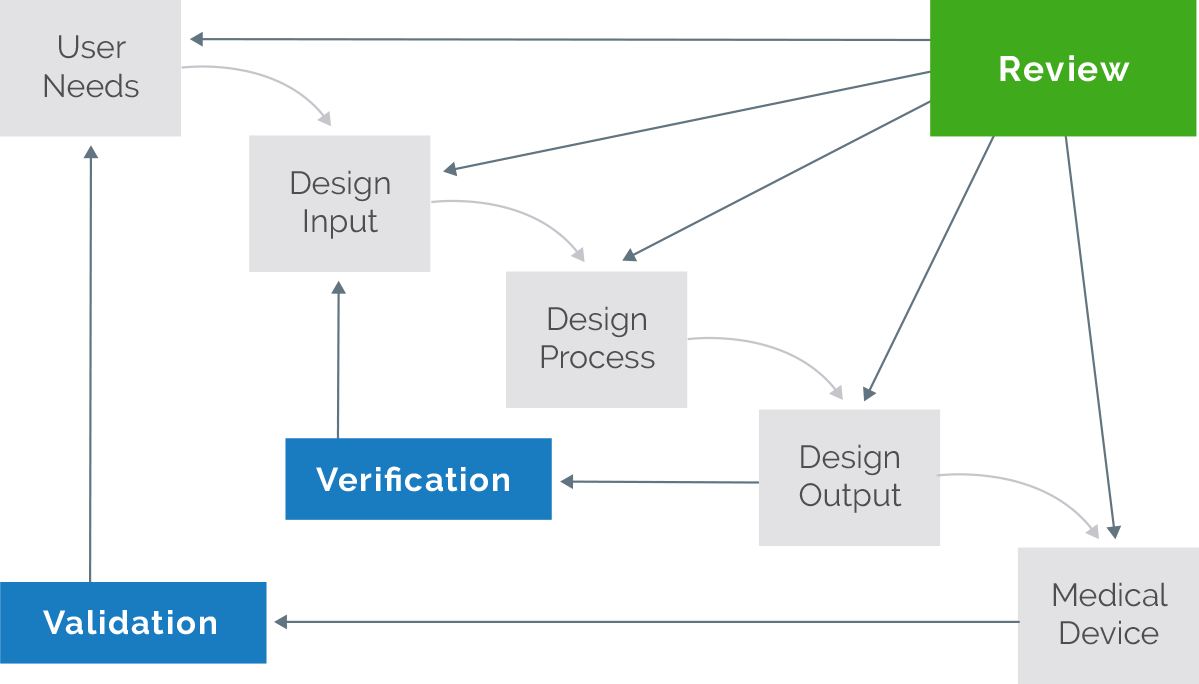
Use Arena QMS for all your design control processes—from development to EU MDR certification/FDA 510(k) clearance, full approval, and production.
Arena’s cloud-native solution accelerates time to value (TTV), delivers seamless, frequent enhancements, and provides startup companies with the most secure, scalable solution so they can realize a fast return on investment (ROI) with the least amount of risk.
Your internal teams and external partners can easily use Arena’s proven and highly configurable solution to keep everyone on the same page. Arena’s purpose-built QMS solutions are designed to improve critical product development and quality management business processes. You won’t need expensive consulting or difficult-to-maintain custom code.
![]()
“In the early stages of any company’s growth, departments often operate in silos focused solely on revenue generation. Tribal knowledge is scattered on napkins, sticky notes, and email chains, leading to inefficiencies and outdated information. Arena has allowed us to bridge the gaps between those siloes and collaborate seamlessly and instantly from design to production to service. This collaboration enhances the quality and compliance of products we put to market, crucial for customers such as ours who are engaged in cutting-edge research and development.”
Ed Engstrom, Quality Assurance Supervisor
![]()
![]()
“I was brought on to evaluate quality processes and systems as we prepared to launch our first product because we didn’t have a system that was robust enough for new product introduction and compliance processes. With Arena QMS, Accuryn has confidence in its quality and compliance processes that couldn’t be realized using manual processes and has a solid, scalable platform to continue its growth and innovation.”
Sanjay Banerjee, COO
![]()
![]()
“We were a fledgling medical device company in the early stages of commercialization and came to Arena from a manual, file-based QMS and PLM environment. We adopted Arena to automate our product development and QMS processes and better manage change control, documentation, training, compliance, and audit processes.”
Mark Templeton, Vice President of Information Technology
![]()
Streamline your product development, quality, and compliance processes and speed product commercialization with Arena QMS.
Get in touch to discuss startup packages!