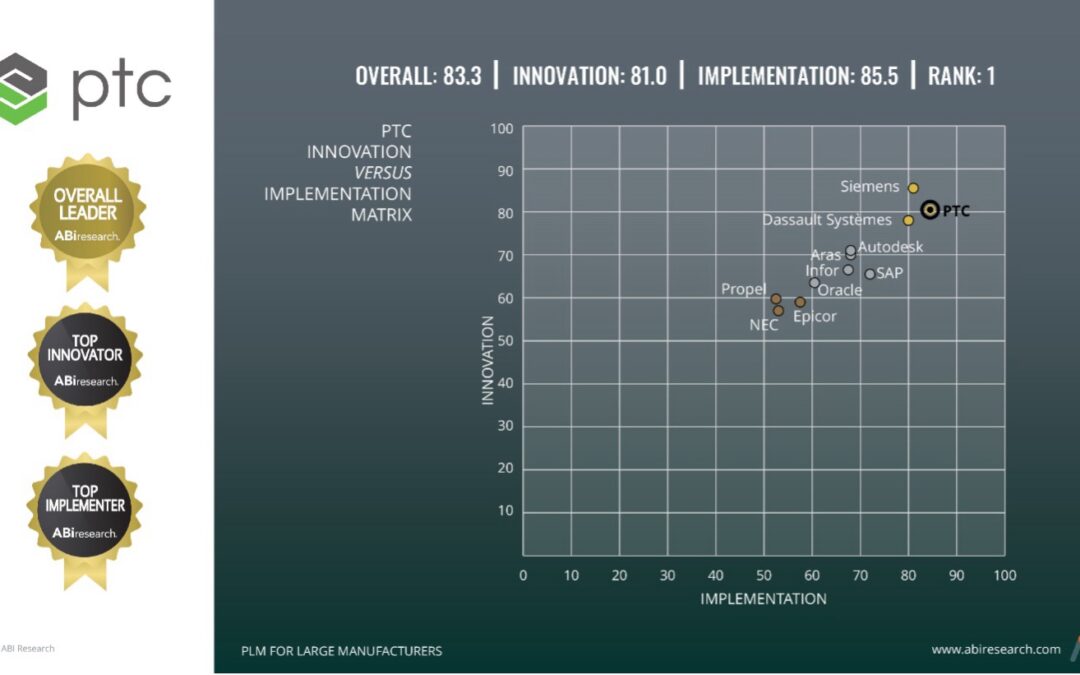
PTC was named the outright leading vendor in ABI Research’s 2024 Enterprise PLM for Large Manufacturing Competitive Assessment. Arena earned high scores in both assessment categories, Innovation and Implementation, ensuring PTC received the #1 vendor score and matrix placement over competitors such as Siemens, Dassault, and Propel. ABI Research’s detailed study assessed and compared 11 PLM...